England is preparing to launch the world's first seven-minute cancer treatment injection
Britain's publicly-funded national healthcare system is set to become a global pioneer by offering a groundbreaking cancer treatment injection to hundreds of patients in England.
The innovative approach has the potential to significantly reduce treatment durations, potentially cutting them down by up to 75%.
Following the green light from the Medicines and Healthcare products Regulatory Agency (MHRA), NHS England announced on Tuesday that hundreds of eligible patients would receive an "under-the-skin" injection of the immunotherapy drug, atezolizumab. This development promises to liberate more time for cancer care teams.
Dr. Alexander Martin, a consultant oncologist at West Suffolk NHS Foundation Trust, remarked, "This approval will not only enhance the convenience and speed of care for our patients but also increase our capacity to treat more patients throughout the day."
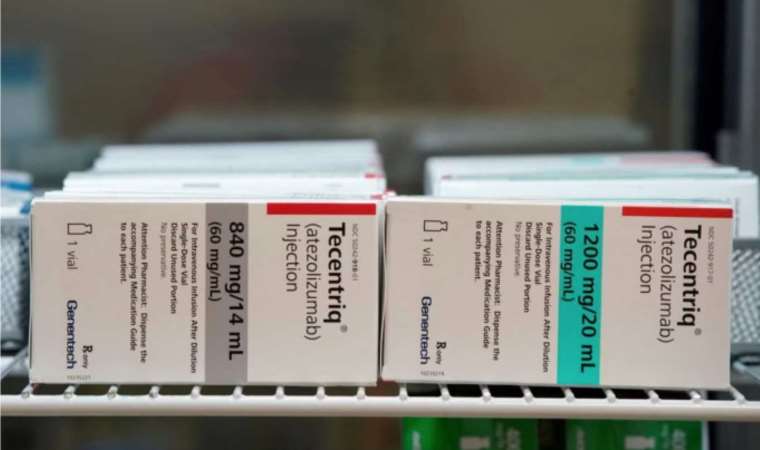
Traditionally, atezolizumab, also known as Tecentriq, has been administered intravenously via a drip, a process that could take anywhere from 30 minutes to an hour, particularly for patients with challenging vein access.
Marius Scholtz, Medical Director at Roche Products Limited, highlighted, "This new approach takes approximately seven minutes, a remarkable improvement compared to the current method of intravenous infusion, which typically takes 30 to 60 minutes."
Atezolizumab, manufactured by Genentech, a subsidiary of Roche (ROG.S), belongs to the category of immunotherapy drugs that bolster a patient's immune system to identify and eradicate cancerous cells. This treatment is currently offered to NHS patients with various cancer types, including lung, breast, liver, and bladder cancers.
NHS England anticipates that the majority of the approximately 3,600 patients who commence atezolizumab treatment annually in England will transition to this time-efficient injection method. However, it is worth noting that patients receiving intravenous chemotherapy alongside atezolizumab may continue to receive transfusions.
